Fuel cell
Fuel cells are a commercially viable alternative for the production of ‘‘clean’’ energy
Fuel cells are a commercially viable alternative for the production of ‘‘clean’’ energy
|
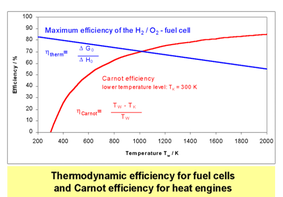
Fuel cells present a highly efficient and environmentally friendly alternative technology for decentralized energy production. Fuel cells have the potential to increase the efficiency without limitation by Carnot cycle as in conventional internal combustion engine and greatly reducing greenhouse gas emissions. Using renewably produced fuels such as hydrogen fuel cells can reduce our dependence on oil, leading to strengthen energy security.
However, the massive use of fuel cells still needs breakthrough research on materials and their interfaces (mainly the electrochemical interfaces). |
Novel materials and novel fuel cell design concepts
Through the development of novel, more active, electrocatalysts aiming to atomic distribution of the metal active phase on stable nanostructured supporting materials and through the synthesis and development of stable anionic alkaline polymer electrolytes, which will allow the use of non precious metal electrocatalysts.
Understanding the functionality and operational characteristics of a 3D structured electrochemical interface.
Aim to increasing utilization of the active electrocatalyst and the innovative design of the flow fields.
Novel designs, engineering and operational concepts can be conceived so as to improve the performance of fuel cells.
Develop advanced integrated approach, based both on materials development and on the deployment of innovative cell designs.
Through the development of novel, more active, electrocatalysts aiming to atomic distribution of the metal active phase on stable nanostructured supporting materials and through the synthesis and development of stable anionic alkaline polymer electrolytes, which will allow the use of non precious metal electrocatalysts.
Understanding the functionality and operational characteristics of a 3D structured electrochemical interface.
Aim to increasing utilization of the active electrocatalyst and the innovative design of the flow fields.
Novel designs, engineering and operational concepts can be conceived so as to improve the performance of fuel cells.
Develop advanced integrated approach, based both on materials development and on the deployment of innovative cell designs.
Microbial Fuel Cell
Microbial fuel cell as new technology for bioelectricity generation
Microbial fuel cell as new technology for bioelectricity generation
|
Microbial fuel cell can be the next generation of fuel cell in energy conservation and alternate fuel utilization. Microbial fuel cell systems can play future role in the area of wastewater purification and electricity production as well as self-sustainable electricity production from algae.
A solar assisted microbial electrolysis cell for hydrogen production driven by a microbial fuel cell can be used for the photo oxidation of organic in wastewater and electricity generation. The process is based on the photoelectroreforming of the organic matter on semiconducting photoanodes and the production of protons H2 which can readily reduce O2 at the cathode for the sustainable electricity production. |
Microbial fuel cells: From fundamentals to applications. A review, Carlo Santoro, Catia Arbizzani, Benjamin Erable, Ioannis Ieropoulos, Journal of Power Sources (2017)
|
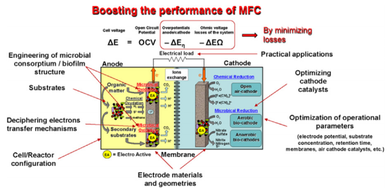
- Electroactive biofilms and electron transfer mechanisms involve with solid electrodes
- Type of material used in electrode preparation will show vital effect on MFCs efficiency. Better performing electrode materials usage will always improve the performance of MFC because different anode materials result in different activation polarization losses.
- Proton exchange electrolyte can affect an MFC system's internal resistance and concentration polarization loss and they in turn influence the power output of the MFC.
- Proton exchange system can affect an MFC system's internal resistance and concentration polarization loss and they in turn influence the power output of the MFC.
- Oxygen is the most commonly used electron acceptor in MFCs for the cathodic reaction. Dissolved oxygen (DO) and oxygen diffusion affects the concentration level of electron acceptors.